Auvi-Q® (epinephrine injection, USP) Companion app for iPhone and iPad
Developer: sanofi-aventis U.S. LLC.
First release : 24 Jan 2013
App size: 26.39 Mb
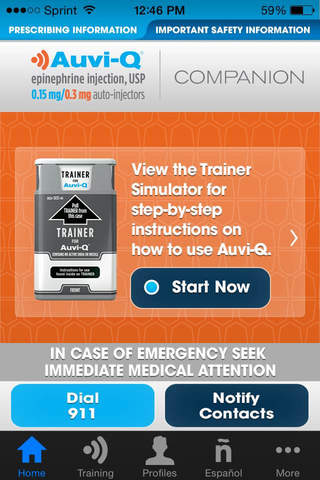
The Auvi-Q® (epinephrine injection, USP) Companion App is designed to help prepare for emergencies. Offering important information about the Auvi-Q device, this app also allows users to create profiles and emergency notification lists.
PROFILES
Keep a profile for every family member with a severe allergy. Add emergency contacts and healthcare provider information too!
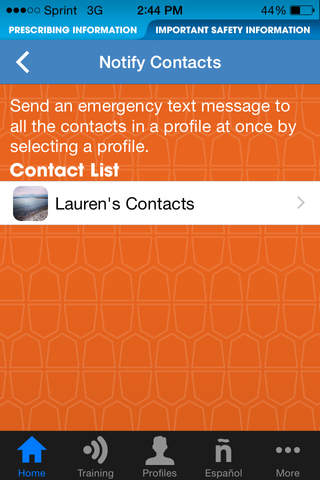
NOTIFY CONTACTS
Send an emergency text message to all the contacts in a profile at once.
AUVI-Q TRAINER SIMULATOR
This simulation demonstrates how Auvi-Q works. We recommend regular practice with the Auvi-Q trainer to make sure you are able to safely use the real Auvi-Q when an allergic emergency happens.
Indication
Auvi-Q® (epinephrine injection, USP) is used to treat life-threatening allergic reactions including anaphylaxis in people who are at risk for or have a history of serious allergic reactions.
Important Safety Information
Auvi-Q is for immediate self (or caregiver) administration and does not take the place of emergency medical care. Seek emergency medical attention immediately after using Auvi-Q. Each Auvi-Q contains a single dose of epinephrine. Auvi-Q should only be injected into your outer thigh. DO NOT INJECT INTO BUTTOCK, OR INTRAVENOUSLY. If you accidentally inject Auvi-Q into any other part of your body, seek emergency medical attention immediately.
Tell your healthcare provider if you have heart disease or are taking certain medicines that can cause heart-related (cardiac) symptoms. Side effects may include an increase in heart rate, a stronger or irregular heartbeat, sweating, nausea and vomiting, difficulty breathing, paleness dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. These side effects usually go away quickly, especially if you rest. If you have high blood pressure or an overactive thyroid, these side effects may be more severe or longer lasting. If you heart disease, you could experience chest pain (angina). If you have diabetes, your blood sugar levels may increase after use. If you have Parkinsons disease, your symptoms may temporarily get worse.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please visit http://products.sanofi.us/auvi-q/auvi-q.html for full prescribing information.
Visit the Sanofi-aventis U.S. LLC Web Site at http://www.sanofi.us/l/us/en/index.jsp
For Auvi-Q® (epinephrine injection, USP) Support visit http://www.auvi-q.com